Biological markers can diagnose Alzheimer's years ahead of clinical symptoms
How different biomarkers can aid in earlier diagnosis and introduce better preventative care
Biomarkers are measurable biological substances; the amount present in the body can be correlated with a condition or a disease. In recent years, researchers have made improvements in identifying biomarkers in Alzheimer’s patients. These findings enable earlier diagnosis, improved treatment options, and more specialized patient care.
For many diseases, a biologically based diagnosis has been standard for decades. Alzheimer’s Disease (AD) has long been diagnosed in part with physical, neurological, and mental status exams. However, cognitive impairment issues can come years after AD takes a biological toll on your body. As neuroscience research progresses, a biological basis for diagnosis is finally becoming standard for neurodegenerative diseases.
Up until recent years, AD was diagnosed using two methods: CSF samples and PET scans.
CSF (cerebrospinal fluid), you may recall from my post last week, is a multifunctional fluid in the brain and spinal cord, used to transport waste, absorb shock, supply nutrients, and as immune protection. CSF samples are collected through a needle in the lower back, with a procedure called a lumbar puncture. These lumbar punctures are minimally invasive, but about 25% of patients experience nausea, vomiting, and headaches that can last up to several days post-operation.
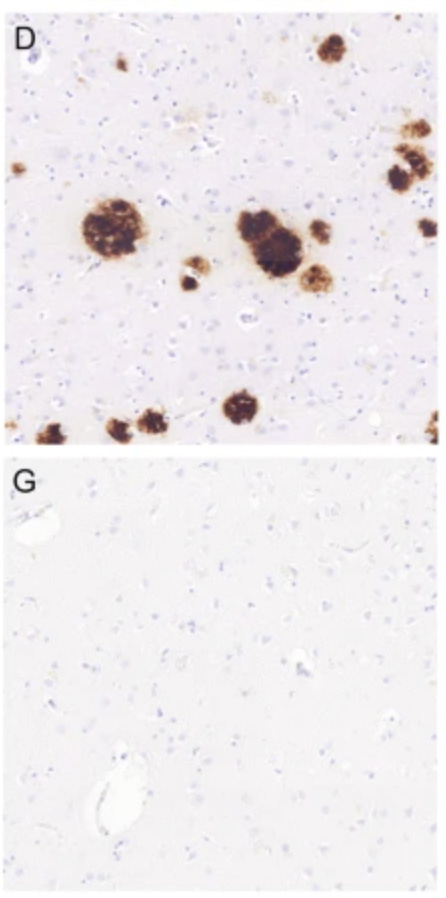
PET (positron emission tomography) scans utilize a radioactive tracer that binds to a specific biomarker. The radiotraced tissue will contrast with the rest of the brain on the scan. As seen below, a healthy amount of an AD-associated biomarker (L), and the amount of this biomarker in several patients with mild AD symptoms (R).
Since 2018, a revolutionary advancement has been made in improving AD diagnostics, the development of blood-based biomarkers (BBM). Simply put, BBMs are biomarkers that can be measured through blood samples. Blood carries plasma, which contains vital proteins, including these biomarkers.
There are two primary biomarkers of Alzheimer’s. The first is amyloid beta (a-beta), a protein that accumulates in gray matter, outside of cells. The precursor to a-beta, amyloid precursor protein, typically functions to strengthen synaptic connections and aid in learning and memory. However, a-beta aggregates into clumps called plaques, which leads to inflammation and cellular damage.
The other primary biomarker in AD is tau, a protein that functions within neurons to provide structural support and cell-to-cell signaling. When chemical changes occur within tau, tau detaches from its supports and aggregates into neurofibrillary tangles. Lack of proper signaling and support leads to a variety of cognitive declines associated with AD.
Although a-beta accumulates first, there is evidence that it forms a feedback loop with tau, where the accumulation of one amplifies the other, and vice versa. This would explain the rapid progression of AD.
The updated 2024 Alzheimer’s Association paper establishes diagnostic measures to characterize stages of AD based on these biomarkers. It combines the presence of beta-amyloid and tau protein clumps, as presented in blood samples, CSF samples, and PET scans. These biological stagings can then be matched and compared with clinical stagings to give a more accurate diagnosis.
Research progression into these biomarkers doesn’t stop at tau and a-beta. The 2024 paper describes several other biomarkers that play a role in dementia and cognitive decline. As scientists continue to understand these complex biological shifts, blood-based tests will aid in the proper differential diagnosis of neurodegenerative diseases.
Standardizing diagnostic measures for AD will generate more straightforward treatment options, based on the staging assigned to the patient. Emerging treatments, such as immunotherapies that target a-beta, can be properly applied based on biomarker staging. Anti-a-beta immunotherapies can reduce amyloid plaque volume and also slow the accumulation of tau protein.
As researchers and clinicians successfully identify and target these protein accumulations, a whole new sector of drug development is forming. Rather than focusing on changing neurotransmitter levels late in AD development, drugs can target these protein buildups early on. Current treatments reduce levels of biomarkers, but this does not imply that they alter the biological mechanisms contributing to the formation of these plaques and tangles.
Stay tuned for future posts to hear about these emerging treatments.
Sources:
Alzheimer Disease: Tau-Positive Neurofibrillary Tangles – Adventures in Neuropathology. (n.d.). Retrieved June 13, 2025, from https://adventuresinneuropathology.com/2018/10/08/alzheimers-disease-tau-positive-neurofibrillary-tangles/
Brain imaging links Alzheimer’s decline to tau protein – WashU Medicine. (n.d.). Retrieved June 13, 2025, from https://medicine.washu.edu/news/brain-imaging-links-alzheimers-decline-to-tau-protein/
Jack, C. R., Andrews, J. S., Beach, T. G., Buracchio, T., Dunn, B., Graf, A., Hansson, O., Ho, C., Jagust, W., McDade, E., Molinuevo, J. L., Okonkwo, O. C., Pani, L., Rafii, M. S., Scheltens, P., Siemers, E., Snyder, H. M., Sperling, R., Teunissen, C. E., & Carrillo, M. C. (2024). Revised criteria for diagnosis and staging of Alzheimer’s disease: Alzheimer’s Association Workgroup. Alzheimer’s & Dementia, 20(8), 5143–5169. https://doi.org/10.1002/alz.13859
Not just amyloid: Physiological functions of the amyloid precursor protein family | Nature Reviews Neuroscience. (n.d.). Retrieved June 13, 2025, from https://www.nature.com/articles/nrn.2017.29
Querol-Vilaseca, M., Colom-Cadena, M., Pegueroles, J., Nuñez-Llaves, R., Luque-Cabecerans, J., Muñoz-Llahuna, L., Andilla, J., Belbin, O., Spires-Jones, T. L., Gelpi, E., Clarimon, J., Loza-Alvarez, P., Fortea, J., & Lleó, A. (2019). Nanoscale structure of amyloid-β plaques in Alzheimer’s disease. Scientific Reports, 9(1), 5181. https://doi.org/10.1038/s41598-019-41443-3